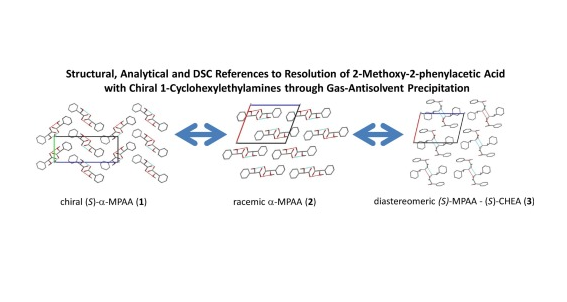
Amit D. Zodge, Petra Bombicz, Edit Székely, György Pokol, János Madarász
Thermochimica Acta, 648, 23-31, (2017)
ABSTRACT
The system of intermolecular interactions is very similar in the solid state of the chiral and racemic α-methoxyphenylacetic acids (α-MPAA) indicated by both Differential Scanning Calorimetry (DSC)and FTIR-spectroscopy, and even proved by single crystal structure determination. It makes the resolution of such a racemate compound challenging even by supercritical fluid xtraction. The chiral(S)-α-methoxyphenylacetic acid (1) crystallizes in orthorhombic crystal system (space group P212121,a = 6.8795(3) Å, b = 7.0124(3) Å, c = 17.4578(8) Å, Z = 4, R = 0.0373), while its 1:1 salt formed with (S)-1-cyclohexylethyl amine (3) s found to be monoclinic (s.g. P21, a = 9.5290(12) Å, b = 6.5909(10) Å,c = 13.869(2) Å, β = 103.13(3)◦, Z = 2, R = 0.0477). The packing arrangement in the (S)-(S) ammonium car-boxylate type salt is columnar. The columns are hydrophylic inward and ydrophobic outward, thatresults in fibrous growth of the salt crystals melting at 163◦C. The crystal habit of the higher meltingdiastereomeric (S)-(R) salt (mp. 187◦C) is also fibrous, its structure is closely related to the (S)-(S)-salt bythe similarities bserved in their IR spectra. The DSC and powder XRD studies on the chiral and racemicacids, and on the pair of diastereomeric salts helped us to construct and calculate the binary and ternaryphase diagrams of the system components, including their utectic temperatures and compositions, aswell.